What you should know about biosimilar drugs
A class of drugs that makes care more affordable has emerged over the last decade.
Advancements in research and technology have resulted in medications made from living organisms to treat many conditions.
Most people know about 2 categories of drugs: brand-name and generic.
In recent years, a third class has come on the scene: biosimilars.
And they’re worth paying attention to.
Biosimilars are not generic drugs. But like generic drugs, biosimilars make medications more affordable.
Sameer Awsare, MD, is an internal medicine physician and associate executive director for The Permanente Medical Group. He partners with national and regional pharmacy leaders at Kaiser Permanente.
As a nationally recognized expert on biosimilars, Dr. Awsare explains what they are and how members and patients can benefit from them.
What are biosimilars? Are they just as good as brand-name drugs?
Biologics are brand-name drugs made from living organisms. Biosimilar drugs are also made from living organisms. They are highly similar versions of the original biologic products.
I want to emphasize that biosimilars are safe and effective. Studies from many countries, including data published by Kaiser Permanente, show biosimilars to be just as safe and effective as brand-name biologics.
As with all medications we use, biosimilars are approved by the Federal Drug Administration.
Tell us a brief history of biosimilars.
The first biosimilar was approved in the United States in 2015, almost 10 years after physicians in Europe began using them. Kaiser Permanente adopted its first biosimilar in 2015. Today, the organization uses 12 biosimilars to treat cancer, rheumatoid arthritis, diabetes, and other conditions.
As of December 2023, the FDA has approved 45 biosimilars. By comparison, Europe has approved twice as many.
Biosimilar uptake has been slower in the U.S. for many reasons. The top barriers are lack of education and misinformation. Patent lawsuits and drug companies offering rebates on brand-name biologics have also slowed adoption.
What’s Kaiser Permanente’s position on biosimilars?
Based on research and evidence, we know that biosimilars are just as safe and effective as biologics. Biosimilars also allow us to improve the affordability of the care we provide.
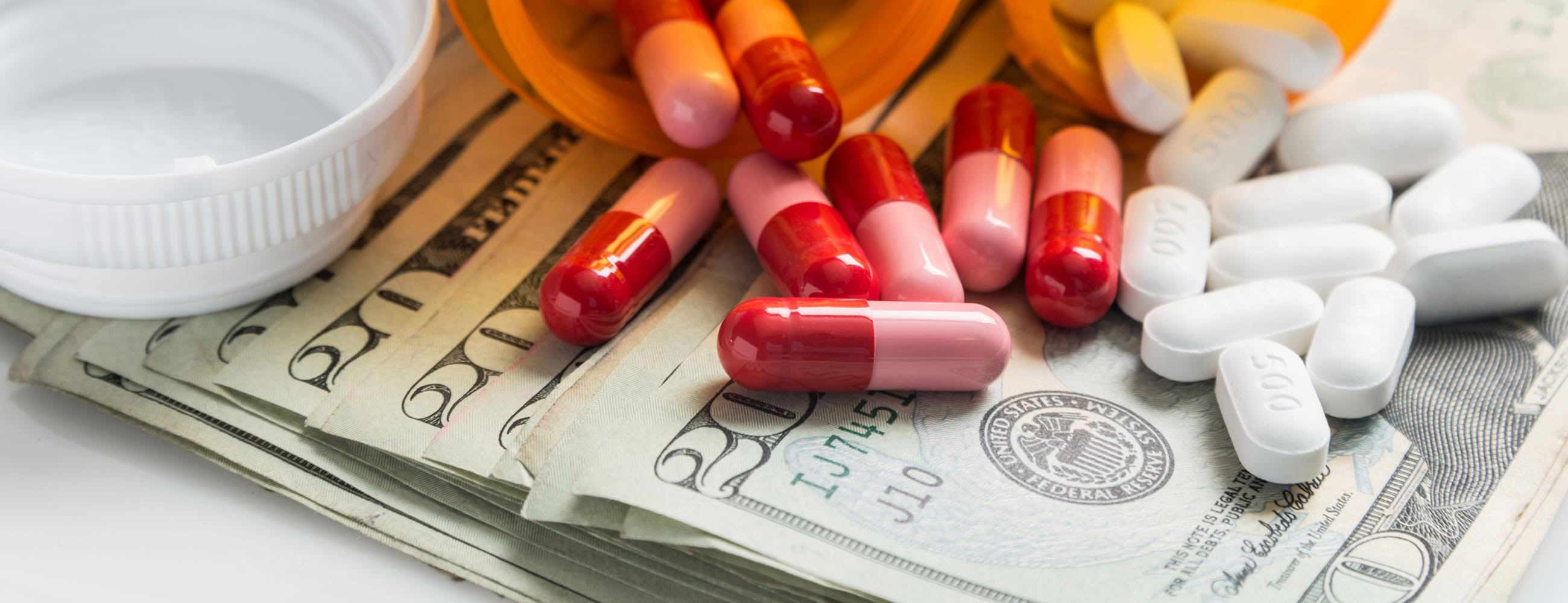
On average, biosimilars cost half as much as biologic equivalents. Biosimilars also create competition by providing alternatives to biologics, driving down biologic drug costs.
Kaiser Permanente is a national leader in the use of biosimilars.
When a new biosimilar becomes available, we use it to treat between 90% to 98% of our members who are medically eligible for it, including those who previously used the original biologic. That is compared to a national new biosimilar usage rate of about 60%.
What makes Kaiser Permanente a leader in biosimilar adoption?
Our integrated care model is the biggest reason we lead in this area.
We have strong communication among patients and physicians. Also, our care teams communicate with each other. Marketing and rebates from biologic manufacturers don’t influence us the way they do many other providers.
We look at the evidence and do what is best and safest for our patients. We don’t face the obstacles like misinformation and rebates that have slowed down others in the American biosimilar market.
Wider use of biosimilars helps lower the cost of health care. Better still, it makes these life-changing drugs available to more patients.
As a physician, I can say giving seriously ill people access to medications that can help treat their illness is what we want for our nation.